In a recent blog, we summarized and discussed two rules recently proposed by CMS. These rules impact Medicare Advantage and Part D plans in a big way, by reshaping quality measures, flexibility, and more. Overall, we’re excited to see more transparency and accessibility in healthcare and feel these rules are moving in the right direction. However, experiences of our own tell us that some further adjustments to the rules may be warranted.
RxAnte’s Response to CMS’s “Flexibility” Rule: CMS-4185-P
Re: Comments to Policy and Technical Changes to the Medicare Advantage, Medicare Prescription Drug Benefit, Program of All-inclusive Care for the Elderly (PACE), Medicaid Fee-For-Service, and Medicaid Managed Care Programs for Years 2020 and 2021 RxAnte is a leading analytics and clinical services organization focused on providing solutions to improve medication use. Founded by nationally recognized subject matter experts in the areas of medication adherence, advanced analytics, and population health, RxAnte’s individual and collective experience spans academia, state and federal government, and industry. The RxAnte team has authored and contributed to over 200 published papers in peer-reviewed journals on the causes and consequences of medication non-adherence, statistical methods related to measuring and predicting medication use, and evaluations of adherence-related interventions. As a core component of our solution, RxAnte partners with MAPD and PDP plans (currently 8.5M lives under management) to improve their Star Rating performance by improving medication use among their membership. RxAnte commends CMS for the release of the Proposed Rule outlining changes to Medicare and Medicaid Programs for Years 2020 and 2021, and appreciates the efforts CMS is taking to gather and incorporate stakeholder opinions to help shape the program and Star Rating technical specifications. In order to provide thoughtful, data-driven feedback on the proposed rule, RxAnte convened an internal team of subject matter experts to include statisticians, clinicians, policy leaders, and strategic account consultants engaging with MAPD health plans. We tested the impact of recommended methodologies in light of the proposed rule using 2017-2019 Part D CMS Stars for MAPD and PDP plans, and also our client MAPD health plan data for the prior 3 years. Overall, RxAnte supports CMS efforts in revisiting the Star Rating cut point methodology for non-CAHPS measures. Consistent with other comments cited, we would prefer cut points that are stable, predictable, and free from the undue influence of outliers. RxAnte urges CMS to consider that plans reallocating resources away from measures where they are already high-performing could be a positive outcome, enabling those plans to focus resources where they could provide the most benefit. Creating predictability in measure cut points would allow plans to prioritize and allocate resources toward improving Star Ratings and calibrate their investment in accordance with what is needed to attain excellent performance. However, RxAnte appreciates the challenge faced by CMS with regard to balancing this desire for predictability with the intent of the cut point methodology, which is to create score groupings that accurately reflect true performance. It is with these challenges and opportunities in mind that RxAnte provides feedback on several specific sections within the proposed rule:
- Mean resampling. In general, RxAnte supports incorporating into the Star Rating cut point methodology an approach that increases the stability of cut points and reduces the impact of outliers. In order to examine the impact of the mean resampling approach, we performed an analysis using this technique on the 2018 and 2019 Stars for MAPD for the three Part D medication adherence measures. We then compared the changes in cut points from 2018 to 2019 Stars using both the current method and the mean resampling method. (We were able to recreate the cut points using the current method for 11 of the 12 cut points.) In eight of the 11 cases, the magnitude of change was the same. Of the remaining three, the magnitude of the change was smaller once and larger twice. Overall, we found that mean resampling had little impact on the cut points, may lead them to be raised more often than lowered, and could run counter to the goal of creating greater stability. Thus, we encourage CMS not to proceed with incorporating mean resampling as a method for calculating cut points.
- Guardrails. As stated above, RxAnte supports the consideration of approaches to increase the stability of measure cut points year over year. In order to examine the impact of the guardrail approach, we tested what the impact of 3% and 5% guardrails would have been for Star Rating years 2017 to 2018 and 2018 to 2019 for the three Part D medication adherence measures. (We did not include Star Rating years 2014-2016 since the year over year changes are not representative of the movement we would expect to see moving forward, given the improved population performance for these Part D measures.) A summary of our findings is below.
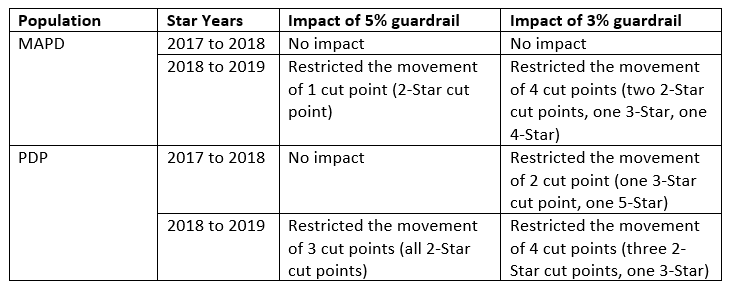
- Based on this analysis, we agree that the incorporation of guardrails would improve cut point stability year over year. While a 3% guardrail would create the greatest stability, we are concerned that it could also lead to a future scenario where CMS would have to “correct” for artificially decreased movement in the cut points created by the guardrail, creating greater overall frustration. Thus, we concur with the CMS recommendation to incorporate a 5% guardrail for cut point changes from one year to the next. Though this will still allow for a significant year over year change, it will provide a slight improvement in stability for cut points in future years.
- Extreme and Uncontrollable Circumstances. RxAnte supports making Star Rating calculation adjustments in extreme and uncontrollable circumstances. We have previously performed analyses that demonstrate how these circumstances can have a negative impact on medication adherence and commend CMS for considering adjustments to Star Ratings accordingly.
- Our analysis examined the influence Hurricane Irma had on medication adherence in a Medicare population. We used a study period between August and October 2017, and defined the pre-period as 25 days prior to and the post-period as the 25 days after landfall (9/10/2017). In this study of 419,139 member-therapies, 211,673 (51%) were geographically located in FEMA affected counties. Average medication supply was significantly reduced for Irma-impacted zip codes post-landfall (-0.9%; SD = 0.4; p<. 05) and was higher than the change seen in non-impacted zip codes (-0.4%; SD = 0.4). Higher-latitude areas experienced significantly less pre vs. post supply disruption on average (-0.8%; SD= 0.4; p<0.05) versus lower latitudes (-1.2%; SD=0.4). Our results suggest naturally occurring disasters can negatively influence adherence rates in affected geographies, specifically by reducing the amount of drug supply patients have on hand directly before and after such an event. Our findings also suggest that the impact of such extreme and uncontrollable circumstances may be highly concentrated to certain zip codes or counties, and may not rise to the level of a state-level state of emergency. We are unclear on whether criteria #s 1 and 2 require a state-level declaration of emergency to qualify selected geographies and contracts as eligible for adjustment. If so, this may be too restrictive. Rather, we find that criteria #3 (minimum percentage of enrollees residing in a FEMA-designated Individual Assistance Area) is most applicable to accurately identify the impact of an uncontrollable event.
- Measure specification updates. RxAnte supports the measure specification updates as described, and further encourages CMS to make measure implementation guides publicly available. We’ve found that the Star Rating Technical Notes allow for variable implementation interpretation for some measures.
- RxAnte identified a recent example in working with health plan clients to improve performance on the Statin Use in Persons with Diabetes (SUPD) measure. The Technical Notes for this measure describe the age criteria as 40-75 years of age and do not include a description of how to determine enrollment. Upon further dialogue with CMS, we’ve learned that the CMS SUPD definition uses member months in calculating the numerator and denominator for the measure, from when a member turns 40 years of age until the month before the member turns 75 years of age. Each episode of enrollment is considered separately when determining numerator and denominator inclusion and exclusion criteria for each measure. Notably, these criteria differ from the PQA measure manual specifications for the measure. We expect this inconsistency has created variability in how this measure is coded and how quality improvement efforts are executed within the industry. An example of the type of implementation guidance that could be useful can be found in the Patient Safety Report User Guide, though this document is currently only accessible via plan sponsors or pharmacy benefit managers (PBMs).
- Telehealth. RxAnte supports the inclusion of telehealth as a basic benefit starting in 2020. As stated, this may assist in remote medication management for patients taking multiple medications and may improve accessibility to opioid addiction treatment for affected individuals in rural communities.
Again, RxAnte appreciates the opportunity to comment and commends CMS for releasing the Proposed Rule outlining changes to Medicare and Medicaid Programs for Years 2020 and 2021. We are available for follow-up clarifications or questions or other practical assistance if requested.
Your Voice Counts: Comment on CMS’s Proposed Rules
The deadline for CMS-4180-P is January 25, 2019, and the deadline for CMS-4185-P is December 31, 2018. We encourage our clients and others in the healthcare community to submit their comments on these rules as well:
- Submit comments on CMS-4180-P: https://www.regulations.gov/comment?D=CMS-2018-0149-0002
- Submit comments on CMS-4185-P: https://www.regulations.gov/comment?D=CMS-2018-0133-0001